KEVZARA offers every-2-week dosing, is available in 2 doses and 2 devices, and can be kept at room temperature (≤77°F) for up to 2 weeks1
Every-2-week-dosing1
The recommended starting dosage for KEVZARA is 200 mg once every 2 weeks, given as a subcutaneous injection for patients with RA1
- No dose adjustments are recommended based on age, gender, race, or weight
- KEVZARA can be used with or without MTX or other conventional DMARD(s)*
- Reduce the dose to 150 mg Q2W for the management of neutropenia, thrombocytopenia, and elevated liver enzymes
Dose modification for patients with RA who need it1
- KEVZARA initiation is not recommended in patients with an ANC <2000 per mm3, platelet count <150,000 per mm3, or ALT or AST >1.5 x ULN1
- If a patient develops a serious infection, hold treatment with KEVZARA until the infection is controlled1
|
RA DOSAGE MODIFICATIONS1 |
|
|
LAB VALUE |
RECOMMENDATION |
|
Low absolute neutrophil count (cells/mm3) |
|
|
ANC >1000 |
Maintain current dosage of KEVZARA. |
|
ANC 500-1000 |
Hold treatment with KEVZARA until ANC >1000. KEVZARA can then be resumed at 150 mg every 2 weeks and increased to 200 mg every 2 weeks as clinically appropriate. |
|
ANC <500 |
Discontinue KEVZARA. |
|
Low platelet count (cells/mm3) |
|
|
50,000-100,000 |
Hold treatment with KEVZARA until platelets >100,000. KEVZARA can then be resumed at 150 mg every 2 weeks and increased to 200 mg every 2 weeks as clinically appropriate. |
|
<50,000 |
If confirmed by repeat testing, discontinue KEVZARA. |
|
Liver enzyme abnormalities |
|
|
ALT or AST >1 to 3x ULN |
Consider dose modification of concomitant DMARDs as clinically appropriate. |
|
ALT or AST >3 to 5x ULN |
Hold treatment with KEVZARA until ALT or AST <3 x ULN. KEVZARA can then be resumed at 150 mg every 2 weeks and increased to 200 mg every 2 weeks as clinically appropriate. |
|
ALT or AST >5x ULN |
Discontinue KEVZARA. |
*Dosing of MTX and other conventional DMARD(s) may vary.1
ALT=alanine aminotransferase; ANC=absolute neutrophil count; DMARDs=disease-modifying antirheumatic drugs; MTX=methotrexate; Q2W=once every 2 weeks; ULN=upper limit of normal.
KEVZARA is available in a pre-filled syringe or a pre-filled, button-free pen1,2
A closer look at the KEVZARA pre-filled, button-free pen1,2
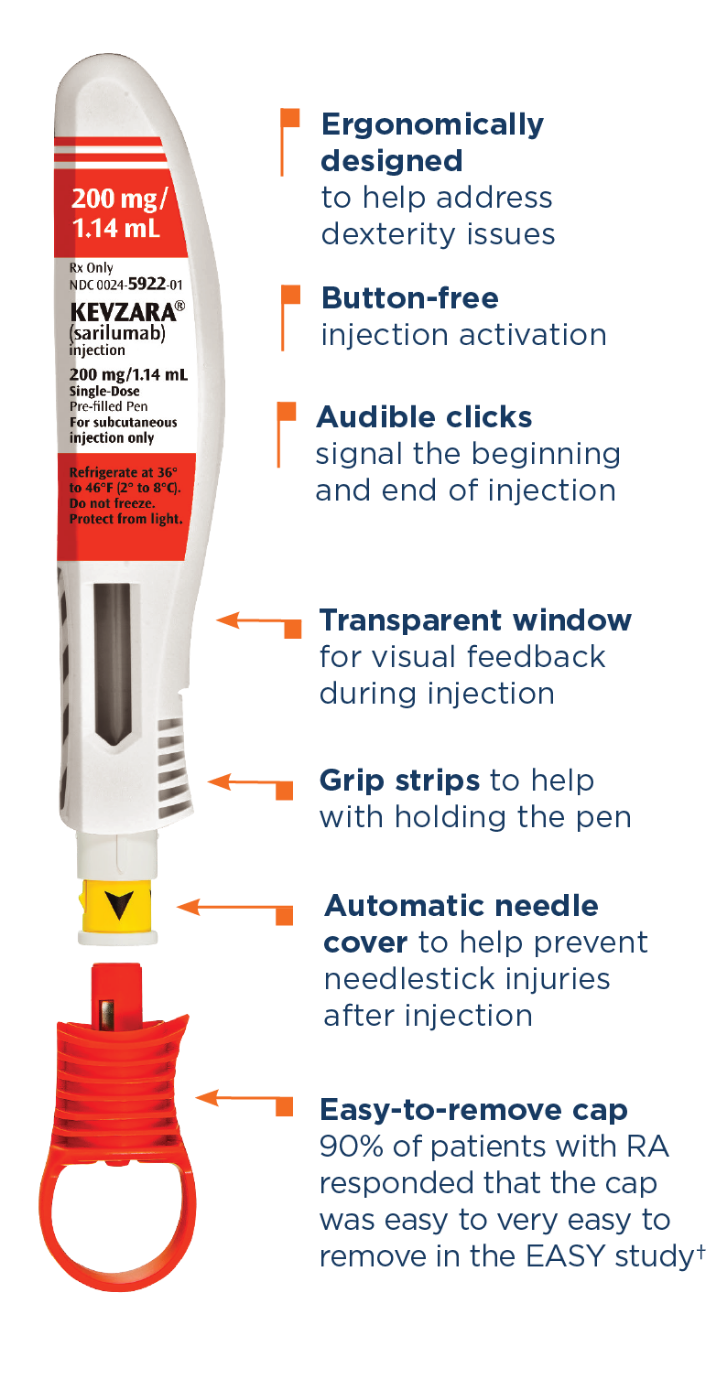
A real-world usability study in patients with RA demonstrated2†‡:
Overall satisfication
98% of patients were satisfied to very satisfied with the KEVZARA pen
Confidence
91% of patients were very to extremely confident that they would be able to give themselves injections with the KEVZARA pen after this study
Easy to use
88% of patients found the KEVZARA pen easy to very easy to use
†55% of patients had past experience with self-injection. All patients were trained on the use of the KEVZARA pen prior to first injection.2
‡EASY Study Description: A 12-week, global, phase 3, randomized, multicenter, open-label study of 217 adult patients with active moderate-to-severe RA designed to assess usability of the KEVZARA pen, of which 108 patients were randomized to the KEVZARA pen. The primary endpoint was defined as number of validated product technical failures (product technical complaint with validated technical cause). One of the secondary objectives was to assess satisfaction with the KEVZARA pen. A total of 600 successful injections were reported during the 12-week study period.2
ALT=alanine aminotransferase; ANC=absolute neutrophil count; AST=aspartate aminotransferase; DMARDs=disease-modifying antirheumatic drugs; MTX=methotrexate; Q2W=once every 2 weeks; RA=rheumatoid arthritis; ULN=upper limit of normal.
The KEVZARA packaging, pre-filled pen, and pre-filled syringe received an Arthritis Foundation Ease of Use Commendation after independent testing by experts and evaluation by people with arthritis. Products receiving the Commendation make certain aspects of life easier for people with RA.3
KEVZARA comes in a pre-filled syringe or a pre-filled, button-free pen. Both should be refrigerated, but either can be kept at room temperature (≤77°F) for up to 2 weeks, if needed.1
IMPORTANT SAFETY INFORMATION
|
WARNING: RISK OF SERIOUS INFECTIONS Patients treated with KEVZARA are at increased risk for developing serious infections that may lead to hospitalization or death. Opportunistic infections have also been reported in patients receiving KEVZARA. Most patients who developed infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids. Avoid use of KEVZARA in patients with an active infection. Reported infections include:
Closely monitor patients for signs and symptoms of infection during treatment with KEVZARA. If a serious infection develops, interrupt KEVZARA until the infection is controlled. Consider the risks and benefits of treatment with KEVZARA prior to initiating therapy in patients with chronic or recurrent infection. |
CONTRAINDICATION
Do not use KEVZARA in patients with known hypersensitivity to sarilumab or any of the inactive ingredients.
WARNINGS AND PRECAUTIONS
- Infections. Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving immunosuppressive agents including KEVZARA. Among opportunistic infections, TB, candidiasis, and pneumocystis were reported with KEVZARA. The most frequently observed serious infections with KEVZARA in RA patients included pneumonia and cellulitis.
- Hold treatment with KEVZARA if a patient develops a serious infection or an opportunistic infection.
- Patients with latent TB should be treated with standard antimycobacterial therapy before initiating KEVZARA. Consider anti-TB therapy prior to initiation of KEVZARA in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent TB but having risk factors for TB infection.
- Consider the risks and benefits of treatment prior to initiating KEVZARA in patients who have: chronic or recurrent infection, a history of serious or opportunistic infections, underlying conditions that may predispose them to infection, been exposed to TB, or lived in or traveled to areas of endemic TB or endemic mycoses.
- Viral reactivation has been reported with immunosuppressive biologic therapies. Cases of herpes zoster were observed in clinical studies with KEVZARA.
- Laboratory Abnormalities. Treatment with KEVZARA was associated with decreases in absolute neutrophil counts (including neutropenia), and platelet counts; and increases in transaminase levels and lipid parameters (LDL, HDL cholesterol, and/or triglycerides). Increased frequency and magnitude of these elevations were observed when potentially hepatotoxic drugs (e.g., MTX) were used in combination with KEVZARA. Assess neutrophil count, platelet count, and ALT/AST levels prior to initiation with KEVZARA. Monitor these parameters 4 to 8 weeks after start of therapy and every 3 months thereafter. Assess lipid parameters 4 to 8 weeks after start of therapy, then at 6 month intervals.
- Gastrointestinal Perforation. GI perforation risk may be increased with concurrent diverticulitis or concomitant use of NSAIDs or corticosteroids. Gastrointestinal perforations have been reported in clinical studies, primarily as complications of diverticulitis. Promptly evaluate patients presenting with new onset abdominal symptoms.
- Immunosuppression. Treatment with immunosuppressants may result in an increased risk of malignancies. The impact of treatment with KEVZARA on the development of malignancies is not known but malignancies have been reported in clinical studies.
- Hypersensitivity Reactions. Hypersensitivity reactions have been reported in association with KEVZARA. Hypersensitivity reactions that required treatment discontinuation were reported in 0.3% of patients in controlled RA trials. Injection site rash, rash, and urticaria were the most frequent hypersensitivity reactions. Advise patients to seek immediate medical attention if they experience any symptoms of a hypersensitivity reaction. If anaphylaxis or other hypersensitivity reaction occurs, stop administration of KEVZARA immediately. Do not administer KEVZARA to patients with known hypersensitivity to sarilumab.
- Active Hepatic Disease and Hepatic Impairment. Treatment with KEVZARA is not recommended in patients with active hepatic disease or hepatic impairment, as treatment with KEVZARA was associated with transaminase elevations.
- Live Vaccines. Avoid concurrent use of live vaccines during treatment with KEVZARA due to potentially increased risk of infections. No data are available on the secondary transmission of infection from persons receiving live vaccines to patients receiving KEVZARA. Prior to initiating treatment, it is recommended that all patients be brought up to date with all immunizations in agreement with current immunization guidelines.
ADVERSE REACTIONS
- For Rheumatoid Arthritis: The most common serious adverse reactions were infections. The most frequently observed serious infections included pneumonia and cellulitis. The most common adverse reactions (occurred in at least 3% of patients treated with KEVZARA + DMARDs) are neutropenia, increased ALT, injection site erythema, upper respiratory infections, and urinary tract infections.
- For Polymyalgia Rheumatica: Serious adverse reactions of neutropenia occurred in 2 patients (3.4%) in the KEVZARA group compared to none in the placebo group. The proportion of patients with serious infections was similar in the KEVZARA group (5.1%) compared to the placebo group (5.2%). The common adverse reactions occurring in ≥5% of patients treated with KEVZARA were neutropenia, leukopenia, constipation, rash pruritic, myalgia, fatigue, and injection site pruritus.
- For Polyarticular Juvenile Idiopathic Arthritis: In Study 4, the rate of infections was 146.6 events per 100 patient-years. The most common infections observed were nasopharyngitis (36.6%) and upper respiratory tract infections (URTI) (14.0%). The most common adverse drug reactions were nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema.
DRUG INTERACTIONS
- Exercise caution when KEVZARA is co-administered with CYP substrates with a narrow therapeutic index (e.g. warfarin or theophylline), or with CYP3A4 substrates (e.g. oral contraceptives or statins) as there may be a reduction in exposure which may reduce the activity of the CYP3A4 substrate.
- Elevated interleukin-6 (IL-6) concentration may down-regulate CYP activity such as in patients with RA and hence increase drug levels compared to subjects without RA. Blockade of IL-6 signaling by IL-6Rα antagonists such as KEVZARA might reverse the inhibitory effect of IL-6 and restore CYP activity, leading to altered drug concentrations.
USE IN SPECIFIC POPULATIONS
- KEVZARA should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus. Because monoclonal antibodies could be excreted in small amounts in human milk, the benefits of breastfeeding and the potential adverse effects on the breastfed child should be considered along with the mother’s clinical need for KEVZARA.
- Use caution when treating the elderly.
Advise patients to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Please see full Prescribing Information, including Boxed WARNING.
INDICATIONS
KEVZARA® (sarilumab) is indicated for treatment of:
- adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs (DMARDs).
- adult patients with polymyalgia rheumatica (PMR) who have had an inadequate response to corticosteroids or who cannot tolerate corticosteroid taper.
- patients who weigh 63 kg or greater with active polyarticular juvenile idiopathic arthritis (pJIA).
IMPORTANT SAFETY INFORMATION
|
WARNING: RISK OF SERIOUS INFECTIONS Patients treated with KEVZARA are at increased risk for developing serious infections that may lead to hospitalization or death. Opportunistic infections have also been reported in patients receiving KEVZARA. Most patients who developed infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids. Avoid use of KEVZARA in patients with an active infection. Reported infections include:
Closely monitor patients for signs and symptoms of infection during treatment with KEVZARA. If a serious infection develops, interrupt KEVZARA until the infection is controlled. Consider the risks and benefits of treatment with KEVZARA prior to initiating therapy in patients with chronic or recurrent infection. |
CONTRAINDICATION
Do not use KEVZARA in patients with known hypersensitivity to sarilumab or any of the inactive ingredients.
WARNINGS AND PRECAUTIONS
- Infections. Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving immunosuppressive agents including KEVZARA. Among opportunistic infections, TB, candidiasis, and pneumocystis were reported with KEVZARA. The most frequently observed serious infections with KEVZARA in RA patients included pneumonia and cellulitis.
- Hold treatment with KEVZARA if a patient develops a serious infection or an opportunistic infection.
- Patients with latent TB should be treated with standard antimycobacterial therapy before initiating KEVZARA. Consider anti-TB therapy prior to initiation of KEVZARA in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent TB but having risk factors for TB infection.
- Consider the risks and benefits of treatment prior to initiating KEVZARA in patients who have: chronic or recurrent infection, a history of serious or opportunistic infections, underlying conditions that may predispose them to infection, been exposed to TB, or lived in or traveled to areas of endemic TB or endemic mycoses.
- Viral reactivation has been reported with immunosuppressive biologic therapies. Cases of herpes zoster were observed in clinical studies with KEVZARA.
- Laboratory Abnormalities. Treatment with KEVZARA was associated with decreases in absolute neutrophil counts (including neutropenia), and platelet counts; and increases in transaminase levels and lipid parameters (LDL, HDL cholesterol, and/or triglycerides). Increased frequency and magnitude of these elevations were observed when potentially hepatotoxic drugs (e.g., MTX) were used in combination with KEVZARA. Assess neutrophil count, platelet count, and ALT/AST levels prior to initiation with KEVZARA. Monitor these parameters 4 to 8 weeks after start of therapy and every 3 months thereafter. Assess lipid parameters 4 to 8 weeks after start of therapy, then at 6 month intervals.
- Gastrointestinal Perforation. GI perforation risk may be increased with concurrent diverticulitis or concomitant use of NSAIDs or corticosteroids. Gastrointestinal perforations have been reported in clinical studies, primarily as complications of diverticulitis. Promptly evaluate patients presenting with new onset abdominal symptoms.
- Immunosuppression. Treatment with immunosuppressants may result in an increased risk of malignancies. The impact of treatment with KEVZARA on the development of malignancies is not known but malignancies have been reported in clinical studies.
- Hypersensitivity Reactions. Hypersensitivity reactions have been reported in association with KEVZARA. Hypersensitivity reactions that required treatment discontinuation were reported in 0.3% of patients in controlled RA trials. Injection site rash, rash, and urticaria were the most frequent hypersensitivity reactions. Advise patients to seek immediate medical attention if they experience any symptoms of a hypersensitivity reaction. If anaphylaxis or other hypersensitivity reaction occurs, stop administration of KEVZARA immediately. Do not administer KEVZARA to patients with known hypersensitivity to sarilumab.
- Active Hepatic Disease and Hepatic Impairment. Treatment with KEVZARA is not recommended in patients with active hepatic disease or hepatic impairment, as treatment with KEVZARA was associated with transaminase elevations.
- Live Vaccines. Avoid concurrent use of live vaccines during treatment with KEVZARA due to potentially increased risk of infections. No data are available on the secondary transmission of infection from persons receiving live vaccines to patients receiving KEVZARA. Prior to initiating treatment, it is recommended that all patients be brought up to date with all immunizations in agreement with current immunization guidelines.
ADVERSE REACTIONS
- For Rheumatoid Arthritis: The most common serious adverse reactions were infections. The most frequently observed serious infections included pneumonia and cellulitis. The most common adverse reactions (occurred in at least 3% of patients treated with KEVZARA + DMARDs) are neutropenia, increased ALT, injection site erythema, upper respiratory infections, and urinary tract infections.
- For Polymyalgia Rheumatica: Serious adverse reactions of neutropenia occurred in 2 patients (3.4%) in the KEVZARA group compared to none in the placebo group. The proportion of patients with serious infections was similar in the KEVZARA group (5.1%) compared to the placebo group (5.2%). The common adverse reactions occurring in ≥5% of patients treated with KEVZARA were neutropenia, leukopenia, constipation, rash pruritic, myalgia, fatigue, and injection site pruritus.
- For Polyarticular Juvenile Idiopathic Arthritis: In Study 4, the rate of infections was 146.6 events per 100 patient-years. The most common infections observed were nasopharyngitis (36.6%) and upper respiratory tract infections (URTI) (14.0%). The most common adverse drug reactions were nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema.
DRUG INTERACTIONS
- Exercise caution when KEVZARA is co-administered with CYP substrates with a narrow therapeutic index (e.g. warfarin or theophylline), or with CYP3A4 substrates (e.g. oral contraceptives or statins) as there may be a reduction in exposure which may reduce the activity of the CYP3A4 substrate.
- Elevated interleukin-6 (IL-6) concentration may down-regulate CYP activity such as in patients with RA and hence increase drug levels compared to subjects without RA. Blockade of IL-6 signaling by IL-6Rα antagonists such as KEVZARA might reverse the inhibitory effect of IL-6 and restore CYP activity, leading to altered drug concentrations.
USE IN SPECIFIC POPULATIONS
- KEVZARA should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus. Because monoclonal antibodies could be excreted in small amounts in human milk, the benefits of breastfeeding and the potential adverse effects on the breastfed child should be considered along with the mother’s clinical need for KEVZARA.
- Use caution when treating the elderly.
Advise patients to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Please see full Prescribing Information, including Boxed WARNING.
INDICATIONS
KEVZARA® (sarilumab) is indicated for treatment of:
- adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs (DMARDs).
- adult patients with polymyalgia rheumatica (PMR) who have had an inadequate response to corticosteroids or who cannot tolerate corticosteroid taper.
- patients who weigh 63 kg or greater with active polyarticular juvenile idiopathic arthritis (pJIA).
References: 1. Kevzara [prescribing information]. Bridgewater, NJ: Sanofi/Regeneron Pharmaceuticals, Inc. 2. Kivitz A, Baret-Cormel L, van Hoogstraten H, et al. Usability and patient preference phase 3 study of the sarilumab pen with active moderate-to-severe rheumatoid arthritis. Rheumatol Ther. 2018;5(1):231-242. 3. KEVZARA® (sarilumab) Administration Options. Arthritis Foundation Web Site. https://www.arthritis.org/partnership/ease-of-use-products/kevzara-button-free-pre-filled-pen. Accessed March 22, 2023.