How to take KEVZARA
What to know about taking KEVZARA
KEVZARA is taken once every 2 weeks in combination with a tapering course of steroids and as monotherapy after tapering and discontinuing treatment with steroids.
Your doctor will guide you on how to gradually lower your dose of steroids. Do not try to inject KEVZARA until you have been shown the right way to give the injections by your healthcare provider.
KEVZARA is given as an injection and is available as either a single-use pre-filled pen or single-use pre-filled syringe. Follow the step-by-step videos and instructions below to learn how to inject KEVZARA using the pre-filled pen or pre-filled syringe.
Sticking to your schedule is important. It may help to think about keeping an “injection calendar” and marking the dates and times of your injections so that you don’t forget.
How to use the pre-filled pen
The KEVZARA pre-filled pen was designed to be comfortably gripped with a cap that’s large and without a button.
Get familiar with using the pre-filled pen
Watch the video for step-by-step instructions on how to use the pre-filled pen.
- moderately to severely active rheumatoid arthritis (RA) after at least one other medicine called a disease-modifying antirheumatic drug (DMARD) has been used and did not work well or could not be tolerated.
- polymyalgia rheumatica (PMR) after corticosteroids have been used and did not work well or when a slow decrease in the dose of corticosteroids (taper) cannot be tolerated.
- SERIOUS INFECTIONS: KEVZARA is a medicine that affects your immune system. KEVZARA can lower the ability of your immune system to fight infections. Some people have had serious infections while using KEVZARA, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections. Your healthcare provider should test you for TB before starting KEVZARA. Your healthcare provider should monitor you closely for signs and symptoms of TB during treatment with KEVZARA.
- Before starting KEVZARA, tell your healthcare provider if you
- think you have an infection or have signs or symptoms of an infection, with or without a fever such as sweats or chills, muscle aches, a cough, shortness of breath, blood in your phlegm, weight loss, warm, red, or painful skin or sores on your body, diarrhea or stomach pain, burning when you urinate or urinating more often than normal, if you feel very tired, or if you are being treated for an infection, get a lot of infections or have repeated infections.
- have diabetes, HIV, or a weakened immune system.
- have TB, or have been in close contact with someone with TB.
- live or have lived, or have traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance of getting certain fungal infections (histoplasmosis, coccidioidomycosis, or blastomycosis).
- have or have had hepatitis.
- After starting KEVZARA, call your healthcare provider right away if you have any symptoms of an infection.
- CHANGES IN CERTAIN LABORATORY TEST RESULTS: Your healthcare provider should do blood tests before and after starting KEVZARA to check for low neutrophil (white blood cells that help the body fight off bacterial infections) counts, low platelet (blood cells that help with blood clotting and stop bleeding) counts, and an increase in certain liver function tests. Changes in test results are common with KEVZARA and can be severe. You may also have changes in other laboratory tests, such as your blood cholesterol levels. Your healthcare provider should do blood tests 4 to 8 weeks after starting KEVZARA and then every 6 months during treatment to check for an increase in blood cholesterol levels.
- TEARS (PERFORATION) OF THE STOMACH OR INTESTINES: Tell your healthcare provider if you have had a condition known as diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines. Some people using KEVZARA get tears in their stomach or intestine. This happens most often in people who also take nonsteroidal anti-inflammatory drugs (NSAIDS), corticosteroids, or methotrexate. Call your healthcare provider right away if you have fever and stomach (abdominal) pain that does not go away.
- CANCER: KEVZARA may increase your risk of certain cancers by changing the way your immune system works. Tell your healthcare provider if you have ever had any type of cancer.
- SERIOUS ALLERGIC REACTIONS: Serious allergic reactions can happen with KEVZARA. Get medical attention right away if you have any of the following signs: shortness of breath or trouble breathing; feeling dizzy or faint; swelling of your lips, tongue, or face; moderate or severe stomach (abdominal) pain or vomiting; or chest pain.
- Do not use KEVZARA if you are allergic to sarilumab or any of the ingredients of KEVZARA.
- Before using KEVZARA, tell your healthcare provider if you
- have an infection.
- have liver problems.
- have had stomach (abdominal) pain or a condition known as diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines.
- recently received or are scheduled to receive a vaccine. People who take KEVZARA should not receive live vaccines.
- All vaccines should be brought up-to-date before starting KEVZARA, unless urgent treatment initiation is required.
- plan to have surgery or a medical procedure.
- are pregnant or plan to become pregnant. It is not known if KEVZARA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Talk to your healthcare provider about the best way to feed your baby if you use KEVZARA. It is not known if KEVZARA passes into your breast milk.
- take prescription or nonprescription medicines, vitamins, or herbal supplements. It is especially important to tell your healthcare provider if you use:
- any other medicines to treat your RA or PMR. Using KEVZARA with these medicines may increase your risk of infection.
- medicines that affect the way certain liver enzymes work. Ask your healthcare provider if you are not sure if your medicine is one of these.
- The most common side effects include:
- injection site redness
- injection site itching
- upper respiratory tract infection
- urinary tract infection
- nasal congestion, sore throat, and runny nose
How to Inject KEVZARA® (sarilumab) Using the Pre-filled Pen
What is KEVZARA?
KEVZARA® (sarilumab) is an injectable prescription medicine called an interleukin-6 (IL-6) receptor blocker. KEVZARA is used to treat adult patients with:
IMPORTANT SAFETY INFORMATION
KEVZARA can cause SERIOUS SIDE EFFECTS including:
Please see additional Important Safety Information throughout this video and full Prescribing Information, including risk of SERIOUS SIDE EFFECTS, and Medication Guide on this website.
Starting a new treatment can be a little overwhelming. Don’t worry, we’re here to help! We’ll walk you through the instructions step by step so you can get more comfortable injecting with the KEVZARA pre-filled pen. With time, it will become part of your routine. You can even set your phone to remind you when it’s time to take KEVZARA.
Before getting started, make sure to read all of the instructions, including the Medication Guide and Instructions for Use, that are in your KEVZARA package.
Watch this entire video to get more familiar with each step and read the Important Safety Information at the end. You can always refer back to the instructions in this video whenever you need.
If your doctor decides you or your caregiver can give KEVZARA at home, they’ll show you how to do it the right way. Don’t try to inject KEVZARA until you’ve been properly trained. You can also ask your doctor if you have questions at any time.
KEVZARA is given as an injection under the skin once every two weeks. You should use the dose prescribed to you by your doctor. The steps for injecting with the pre-filled pen are the same, no matter which dose your doctor prescribed.
Here are a few things to remember: You should store the KEVZARA pre-filled pen inside its original carton in your refrigerator between 36 degrees Fahrenheit and 46 degrees Fahrenheit until you’re ready to use it. Keep the pre-filled pen out of the reach of children.
Do not freeze, heat up, shake, or expose the pre-filled pen to direct sunlight.
Once you take the pre-filled pen out of the refrigerator, it can be stored at room temperature up to 77 degrees Fahrenheit for up to 14 days in the original packaging. Throw away the pre-filled pen if it has been kept at room temperature and was not used within 14 days. Keep KEVZARA and all medicines out of the reach of children.
Don’t use the pre-filled pen if it’s damaged or the orange cap is missing or not attached. If it is, return the unused pen and the package it came in to your pharmacy. Don’t ever reuse the pre-filled pen and don’t try to inject KEVZARA through your clothes.
Now, let’s go through the instructions for injecting. You can use the Instructions for Use that came with your prescription to follow along.
Before you get started, prepare everything you need on a clean, flat surface.
You’ll need an unused KEVZARA pre-filled pen. You’ll also need an alcohol wipe, a cotton ball or gauze, and a sharps disposal container.
Take one of your pens out of the packaging by holding the middle of the pen body. Don’t remove the orange cap until you are ready to inject. If you have another pen left in the package, put it back in the refrigerator now.
Look at the label and check to see that you have the right dose. Also, check the expiration date. You will see this labeled on the side of the pen. If the expiration date has passed, don’t use it. Throw it away in your sharps container and use a new one.
Next, take a look at the window of the pre-filled pen. If the liquid in the window is clear and colorless to pale yellow, you’re good to go. You may see air bubbles, but this is normal.
However, if the liquid in the window is cloudy, discolored or contains particles, or if the window is solid yellow, don’t use it. Throw it away in your sharps container and get a new one.
Now, you’ll need to lay the pen on a flat surface and let it warm up to room temperature. This should take at least 60 minutes. Using the pen at room temperature may help make the injection more comfortable. Don’t try to warm the pen in any other way. Let it warm up naturally. Don’t use the pen if it’s been out of the refrigerator for more than 14 days.
The next step is choosing where to inject. You can inject into your thigh or belly. Try not to get too close to your belly button by staying more than 2 inches, or 5 centimeters, away from your belly button.
If someone else is giving you the injection, they can use the outer area of your upper arm.
Be sure to change the injection site each time you inject, and don’t inject into skin that is tender, damaged, or has bruises or scars.
The last step before injecting is preparing your injection site. Make sure you wash your hands with soap and water before you do this.
After washing your hands, use an alcohol wipe to clean the skin at the injection site really well and then let it air dry. Don’t touch the injection site again before the injection.
Now you’re ready to give the injection. You should only perform this part after completing all the steps in the “Get ready for your injection” section.
You will start by twisting or pulling off the orange cap. Don’t touch the yellow needle cover and don’t put the orange cap back on.
Next, you will put the yellow needle cover on your skin at about a 90-degree angle. Make sure you can see the window.
Press down and hold the pen firmly against your skin. There will be a click when the injection starts.
Keep holding the pen firmly against your skin. You will see the window starting to turn solid yellow. The injection can take up to 15 seconds.
You will hear a second click when the injection is complete. Check to see that the entire window has turned solid yellow before you remove the pen. If you do not hear the second click, you should still check to see if the entire window has turned solid yellow.
If the entire window does not turn solid yellow, this could mean that you may not have received your full dose of medicine. If this happens, call your doctor right away.
Now, you can remove the pen from your skin. The needle will be covered automatically after you remove the pen from your skin.
You might see a little bleeding at the injection site. Don’t worry, just press a cotton ball or gauze over the site until the bleeding stops, but don’t rub it.
Throw away the pen in a sharps disposal container right after you finish using it. Don’t throw the pen out in your household trash or put the orange cap back on, and please do not reuse it.
If you don’t have an FDA-cleared sharps disposal container, you may use a puncture-resistant container that is made of a heavy-duty plastic that can be closed with a tight-fitting, puncture-resistant lid, to prevent the sharps from being able to come out.
The container should be upright and stable during use, leak-resistant, and properly labeled to warn people that there’s hazardous waste inside.
Also, don’t recycle your used sharps container or throw your used sharps container in the trash unless your community guidelines permit it. You can find more information about safely disposing used sharps containers, including specific information about sharps disposal in the state you live in, by visiting the FDA website at www.fda.gov/safesharpsdisposal.
Remember to always keep the sharps disposal container and the KEVZARA pre-filled pen out of the reach of children.
If you have any questions or concerns about your medication or your disease, always call your doctor. Please continue watching for additional Important Safety Information and see the full Prescribing Information, including risk of SERIOUS SIDE EFFECTS, and Medication Guide on this website.
IMPORTANT SAFETY INFORMATION (CONTINUED)
These are not all the possible side effects of KEVZARA. Tell your doctor about any side effect that bothers you or does not go away. You are encouraged to report side effects of prescription drugs to the FDA at www.fda.gov/medwatch or call 1-800-FDA-1088.
To learn more, talk about KEVZARA with your healthcare provider or pharmacist. The FDA-approved Medication Guide and Prescribing Information can be found at www.KEVZARA.com or by calling 1-844-KEVZARA.
Please see full Prescribing Information, including risk of SERIOUS SIDE EFFECTS, and Medication Guide on this website.
There you have it! We hope this video was helpful for you as you get comfortable with injecting using the KEVZARA pre-filled pen.
Should you ever need to refresh your memory, you can re-watch this video at any time. You can also find the step-by-step instructions at www.KEVZARA.com.
Follow the step-by-step instructions
-
- Read all of the instructions carefully before using the pre-filled pen
- Keep unused pens in the original carton and store in the refrigerator between 36°F and 46°F (2°C and 8°C)
- Keep the carton in an insulated bag with an ice pack when traveling
- Let the pen warm up at room temperature for at least 60 minutes before using
- Use the pen within 14 days after taking it out of the refrigerator or insulated bag
- Keep the pen and all medicines out of the reach of children
- Read all of the instructions carefully before using the pre-filled pen
- Keep unused pens in the original carton and store in the refrigerator between 36°F and 46°F (2°C and 8°C)
- Keep the carton in an insulated bag with an ice pack when traveling
- Let the pen warm up at room temperature for at least 60 minutes before using
- Use the pen within 14 days after taking it out of the refrigerator or insulated bag
- Keep the pen and all medicines out of the reach of children
-
- Do not use the pre-filled pen if it has been damaged or if the orange cap is missing or not attached. Return the pen and the package it came in to your pharmacy
- Do not remove the orange cap until just before you are ready to inject
- Do not touch the yellow needle cover
- Do not try to put the orange cap back on the pen
- Do not reuse the pen
- Do not freeze or heat up the pen
- Do not expose the pen to direct sunlight
- Do not inject through your clothes
- Do not use the pre-filled pen if it has been damaged or if the orange cap is missing or not attached. Return the pen and the package it came in to your pharmacy
- Do not remove the orange cap until just before you are ready to inject
- Do not touch the yellow needle cover
- Do not try to put the orange cap back on the pen
- Do not reuse the pen
- Do not freeze or heat up the pen
- Do not expose the pen to direct sunlight
- Do not inject through your clothes
This subcutaneous injection should be taken once every 2 weeks.
What you’ll need for your injection:
An unused KEVZARA pre-filled pen
Alcohol wipe
Cotton ball or gauze
Sharps disposal container
Step A: Get ready for an injection

Take 1 pen out of the packaging by holding the middle of the pen body
Do not remove the orange cap until you are ready to inject
Keep the remaining pens in the carton in the refrigerator
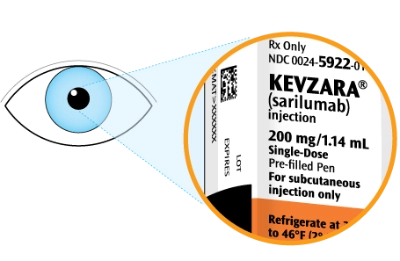
Check that you have the correct medicine and the correct dose
Check the expiration date. This is shown on the side of the pen
Do not use the pen if the expiration date has passed
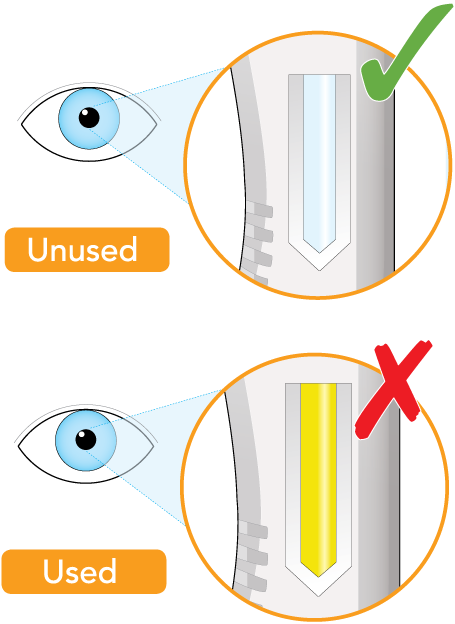
Check that the liquid in the window is clear and colorless to pale yellow
You may see air bubbles. This is normal
Do not inject if the liquid is cloudy, discolored, or contains particles. Safely throw away (dispose of) the pen in a sharps container and get a new one
The window is clear when the pen is unused. The window will turn solid yellow after the pen has been used
Do not use if the window is solid yellow. Safely dispose of the pen in a sharps container and get a new one
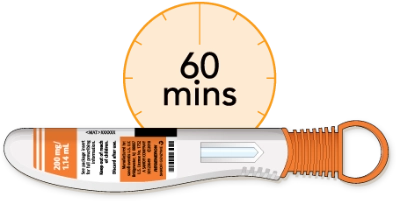
Using the pen at room temperature may make the injection more comfortable
Do not use the pen if it has been out of the refrigerator for more than 14 days
Do not try to warm the pen in any other way
You can inject into your thigh or belly (abdomen), except for the area 2 inches (5 cm) around your belly button (navel)
If somebody else gives you the injection, they can also use the outer area of the upper arm
Change the injection site each time you inject
Do not inject into skin that is tender, damaged, or has bruises or scars
Wash your hands
Clean the skin at the injection site with an alcohol wipe and let it air dry before injecting
Do not touch the injection site again before injection
Step B: Give the injection
(Complete Step B after completing all steps in Step A)
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.

Do not remove the orange cap until you are ready to inject
Do not touch the yellow needle cover
Do not put the orange cap back on
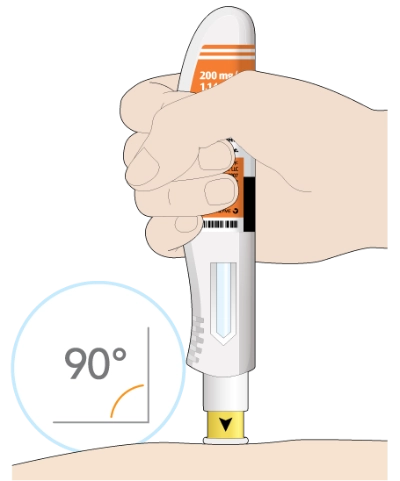
Make sure you can see the window

There will be a “click” when the injection starts
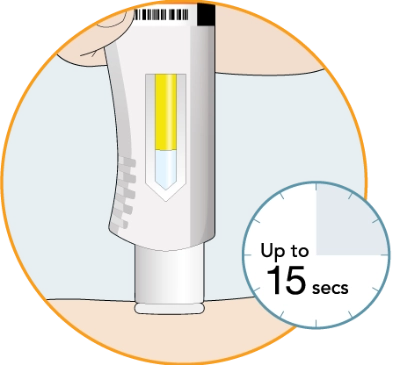
The window will start to turn solid yellow
The injection can take up to 15 seconds

If you do not hear the second click, you should still check to see if the entire window has turned solid yellow
If the entire window does not turn solid yellow, this means that you may not have received your full dose of medicine. Call your healthcare provider right away
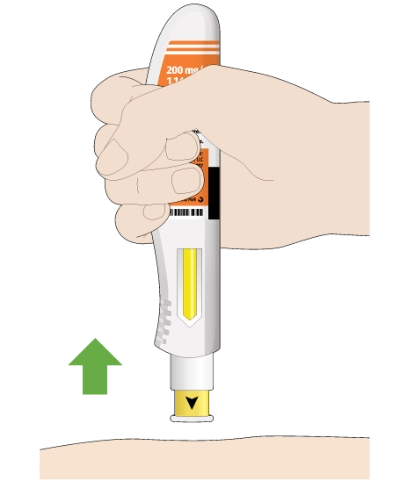
After you remove the pen from your skin, the needle will be covered automatically
If you see any blood at the injection site, press a cotton ball or gauze on the site
Do not rub the injection site
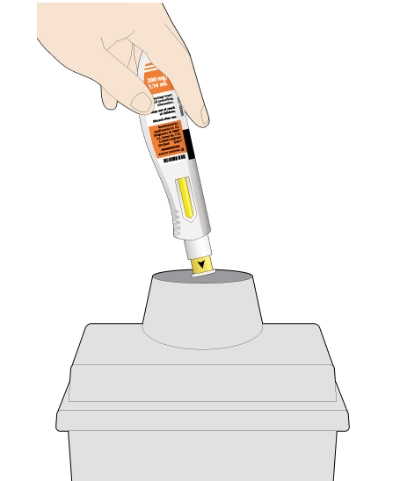
The KEVZARA pre-filled pen should not be reused
Put the used pen into your FDA-cleared sharps disposal container or a puncture-resistant container
Do not put the orange cap back on
How should I throw away (dispose of) KEVZARA pre-filled pens?
Put the used pen in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the pen in your household trash
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
Do not reuse the pen
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Important: Always keep the sharps disposal container out of the reach of children.
Keep KEVZARA cool
When you fill your prescription for KEVZARA, it’s important to keep it in your refrigerator. But once it reaches room temperature, you’ll have to use the pen within 14 days or throw it away.

How to use the pre-filled syringe
Some people feel more comfortable with the idea of injecting with a syringe. That’s why KEVZARA also comes in a pre-filled syringe.
Get familiar with using the pre-filled syringe
Watch the video for step-by-step instructions on how to use the pre-filled syringe.
- adult patients with moderately to severely active rheumatoid arthritis (RA) after at least one other medicine called a disease-modifying antirheumatic drug (DMARD) has been used and did not work well or could not be tolerated.
- adult patients with polymyalgia rheumatica (PMR) after corticosteroids have been used and did not work well or when a slow decrease in the dose of corticosteroids (taper) cannot be tolerated.
- people with active polyarticular juvenile idiopathic arthritis (pJIA) who weigh 63kg (139 lbs) or more.
- SERIOUS INFECTIONS. KEVZARA is a medicine that affects your immune system. KEVZARA can lower the ability of your immune system to fight infections. Some people have had serious infections while using KEVZARA, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections. Your healthcare provider should test you or your child for TB before starting KEVZARA. Your healthcare provider should monitor you closely for signs and symptoms of TB during treatment with KEVZARA.
- Before starting KEVZARA, tell your healthcare provider if you:
- think you have an infection or have signs or symptoms of an infection, with or without a fever such as: sweats or chills, muscle aches, a cough, shortness of breath, blood in your phlegm, weight loss, warm, red, or painful skin or sores on your body, diarrhea or stomach pain, burning when you urinate or urinating more often than normal, if you feel very tired, or if you are being treated for an infection, get a lot of infections or have repeated infections.
- have diabetes, HIV, or a weakened immune system.
- have TB, or have been in close contact with someone with TB.
- live or have lived, or have traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance of getting certain fungal infections (histoplasmosis, coccidioidomycosis, or blastomycosis).
- have or have had hepatitis.
- After starting KEVZARA, call your healthcare provider right away if you have any symptoms of an infection.
- CHANGES IN CERTAIN LABORATORY TEST RESULTS: Your or your child’s healthcare provider should do blood tests before and after starting KEVZARA to check for low neutrophil (white blood cells that help the body fight off bacterial infections) counts, low platelet (blood cells that help with blood clotting and stop bleeding) counts, and an increase in certain liver function tests. Changes in test results are common with KEVZARA and can be severe. You may also have changes in other laboratory tests, such as your blood cholesterol levels. Your or your child’s healthcare provider should do blood tests 4 to 8 weeks after starting KEVZARA and then every 6 months during treatment to check for an increase in blood cholesterol levels.
- TEARS (PERFORATION) OF THE STOMACH OR INTESTINES: Tell your healthcare provider if you have had a condition known as diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines. Some people using KEVZARA get tears in their stomach or intestine. This happens most often in people who also take nonsteroidal anti-inflammatory drugs (NSAIDS), corticosteroids, or methotrexate. Call your healthcare provider right away if you have fever and stomach (abdominal) pain that does not go away.
- CANCER: KEVZARA may increase your risk of certain cancers by changing the way your immune system works. Tell your healthcare provider if you have ever had any type of cancer.
- SERIOUS ALLERGIC REACTIONS: Serious allergic reactions can happen with KEVZARA. Get medical attention right away if you or your child have any of the following signs: shortness of breath or trouble breathing; feeling dizzy or faint; swelling of your lips, tongue, or face; moderate or severe stomach (abdominal) pain or vomiting; or chest pain.
- Do not use KEVZARA if you or your child are allergic to sarilumab or any of the ingredients of KEVZARA.
- Before you or your child use KEVZARA, tell your healthcare provider if you or your child:
- have an infection.
- have liver problems.
- have had stomach (abdominal) pain or a condition known as diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines.
- recently received or are scheduled to receive a vaccine. People who take KEVZARA should not receive live vaccines.
- All vaccines should be brought up-to-date before starting KEVZARA, unless urgent treatment initiation is required.
- plan to have surgery or a medical procedure.
- are pregnant or plan to become pregnant. It is not known if KEVZARA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Talk to your healthcare provider about the best way to feed your baby if you use KEVZARA. It is not known if KEVZARA passes into your breast milk.
- take prescription or nonprescription medicines, vitamins, or herbal supplements. It is especially important to tell your healthcare provider if you or your child use:
- any other medicines to treat your RA, PMR, or pJIA. Using KEVZARA with these medicines may increase your risk of infection.
- medicines that affect the way certain liver enzymes work. Ask your healthcare provider if you are not sure if your medicine is one of these.
- The most common side effects include:
- injection site redness
- injection site itching
- upper respiratory tract infection
- urinary tract infection
- nasal congestion, sore throat, and runny nose
How to Inject KEVZARA® (sarilumab) Using the Pre-filled Syringe
What is KEVZARA?
KEVZARA® (sarilumab) is an injectable prescription medicine called an interleukin-6 (IL-6) receptor blocker. KEVZARA is used to treat:
It is not known if KEVZARA is safe and effective in children with pJIA under 2 years of age.
IMPORTANT SAFETY INFORMATION
KEVZARA can cause SERIOUS SIDE EFFECTS including:
Please see additional Important Safety Information throughout this video and full Prescribing Information, including risk of SERIOUS SIDE EFFECTS, and Medication Guide on this website.
Starting a new treatment can be a little overwhelming. Don’t worry, we’re here to help! We’ll walk you through the instructions step by step so you can get more comfortable injecting with the KEVZARA pre-filled syringe. With time, it will become part of your routine. You can even set your phone to remind you when it’s time to take KEVZARA.
Before getting started, make sure to read all of the instructions, including the Medication Guide and Instructions for Use, that are in your KEVZARA package.
Watch this entire video to get more familiar with each step and read the Important Safety Information at the end. You can always refer back to the instructions in this video whenever you need.
If your doctor decides you or your caregiver can give KEVZARA at home, they’ll show you how to do it the right way. Don’t try to inject KEVZARA until you’ve been properly trained. You can also ask your doctor if you have questions at any time.
KEVZARA is given as an injection under the skin once every two weeks. You should use the dose prescribed to you by your doctor. The steps for injecting with the pre-filled syringe are the same, no matter which dose your doctor prescribed.
Here are a few things to remember: You should store the KEVZARA pre-filled syringe inside its original carton in your refrigerator between 36 degrees Fahrenheit and 46 degrees Fahrenheit until you’re ready to use it. Keep the pre-filled syringe out of the reach of children.
Do not freeze, heat up, shake, or expose the pre-filled syringe to direct sunlight.
Once you take the pre-filled syringe out of the refrigerator, it can be stored at room temperature up to 77 degrees Fahrenheit for up to 14 days in the original packaging. Throw away the pre-filled syringe if it has been kept at room temperature and was not used within 14 days. Keep KEVZARA and all medicines out of the reach of children.
Don’t use the pre-filled syringe if it is damaged or the needle cap is missing or not attached. If it is, return the unused syringe and the package it came in to your pharmacy. Don’t ever reuse the pre-filled syringe and don’t try to inject KEVZARA through your clothes.
Now, let’s go through the instructions for injecting. You can use the Instructions for Use that came with your prescription to follow along.
Before you get started, prepare everything you need on a clean, flat surface.
You’ll need an unused KEVZARA pre-filled syringe. You’ll also need an alcohol wipe, a cotton ball or gauze, and a sharps disposal container.
Take one of your syringes out of the packaging by holding the middle of the syringe body. Don’t hold the syringe by the plunger, finger grip, or needle cap. Don’t pull off the needle cap until you are ready to inject. If you have another syringe left in the package, put it back in the refrigerator now.
Look at the label and check to see that you have the right dose. Also, check the expiration date. You will see this labeled on the side of the syringe. If the expiration date has passed, don’t use it. Throw it away in your sharps container and get a new one.
Next, take a look at the liquid in the syringe. If the liquid is clear and colorless to pale yellow, you’re good to go. You may see air bubbles, but this is normal.
However, if the liquid is cloudy, discolored, or contains particles, don’t use it. Throw it away in your sharps container and get a new syringe.
Now, you’ll need to lay the syringe on a flat surface and let it warm up to room temperature. This should take at least 30 minutes. Using the syringe at room temperature may help make the injection more comfortable. Don’t try to warm it up in any other way. Let it warm up naturally. Don’t use the syringe if it’s been out of the refrigerator for more than 14 days.
The next step is choosing where to inject. You can inject into your thigh or belly. Try not to get too close to your belly button by staying more than 2 inches, or 5 centimeters, away from your belly button.
If someone else is giving you the injection, they can use the outer area of your upper arm.
Be sure to change the injection site each time you inject, and don’t inject into skin that is tender, damaged, or has bruises or scars.
The last step before injecting is preparing your injection site. Make sure you wash your hands with soap and water before you do this.
After washing your hands, use an alcohol wipe to clean the skin at the injection site really well and then let it air dry. Don’t touch the injection site again before the injection.
Now you’re ready to give the injection. You should only perform this part after completing all the steps in the “Get ready for your injection” section.
First, pull off the needle cap by holding the syringe at the middle of the syringe body with the needle pointing away from you. Don’t remove the needle cap until you are ready to inject. Keep your hands off the plunger. If you notice air bubbles, that’s OK. Don’t try to get rid of any air bubbles in the syringe before injecting.
Next, using your thumb and index finger, pinch a fold of skin at the clean injection site without touching the site itself.
Now, take a deep breath, exhale… and insert the needle into the fold of your skin at about a 45-degree angle.
Slowly push the plunger down as far as it will go until the syringe is empty.
Once the plunger is down, make sure to check that the syringe is empty. Then pull the needle out at the same angle it went in.
You might see a little bleeding at the injection site. Don’t worry, just press a cotton ball or gauze over the site until the bleeding stops, but don’t rub it.
Throw away the syringe in a sharps disposal container right after you finish using it. Don’t throw the syringe out in your household trash or put the needle cap back on, and never reuse a syringe.
If you don’t have an FDA-cleared sharps disposal container, you may use a puncture-resistant container that is made of a heavy-duty plastic that can be closed with a tight-fitting, puncture-resistant lid, to prevent the sharps from being able to come out.
The container should be upright and stable during use, leak-resistant, and properly labeled to warn people that there’s hazardous waste inside.
Also, don’t recycle your used sharps container or throw your used sharps container in the trash unless your community guidelines permit it. You can find more information about safely disposing used sharps containers, including specific information about sharps disposal in the state you live in, by visiting the FDA website at www.fda.gov/safesharpsdisposal.
Remember to always keep the sharps disposal container and the KEVZARA pre-filled syringe out of the reach of children.
If you have any questions or concerns about your medication or your disease, always call your doctor. Please continue watching for additional Important Safety Information and see the full Prescribing Information, including risk of SERIOUS SIDE EFFECTS, and Medication Guide on this website.
IMPORTANT SAFETY INFORMATION (CONTINUED)
These are not all the possible side effects of KEVZARA. Tell your doctor about any side effect that bothers you or does not go away. You are encouraged to report side effects of prescription drugs to the FDA at www.fda.gov/medwatch or call 1-800-FDA-1088.
To learn more, talk about KEVZARA with your healthcare provider or pharmacist. The FDA-approved Medication Guide and Prescribing Information can be found at www.KEVZARA.com or by calling 1-844-KEVZARA.
Please see full Prescribing Information, including risk of SERIOUS SIDE EFFECTS, and Medication Guide on this website.
There you have it! We hope this video was helpful for you as you get comfortable with injecting using the KEVZARA pre-filled syringe.
Should you ever need to refresh your memory, you can re-watch this video at any time. You can also find the step-by-step instructions at www.KEVZARA.com.
Follow the step-by-step instructions
-
- Read all of the instructions carefully before using the pre-filled syringe
- Keep unused syringes in the original carton and store in the refrigerator between 36°F and 46°F (2°C and 8°C)
- Keep the carton in an insulated bag with an ice pack when traveling
- Let the syringe warm up at room temperature for at least 30 minutes before using
- Use the syringe within 14 days after taking it out of the refrigerator or insulated bag
- Keep the syringe and all medicines out of the reach of children
- Read all of the instructions carefully before using the pre-filled syringe
- Keep unused syringes in the original carton and store in the refrigerator between 36°F and 46°F (2°C and 8°C)
- Keep the carton in an insulated bag with an ice pack when traveling
- Let the syringe warm up at room temperature for at least 30 minutes before using
- Use the syringe within 14 days after taking it out of the refrigerator or insulated bag
- Keep the syringe and all medicines out of the reach of children
-
- Do not use the pre-filled syringe if it has been damaged or if the needle cap is missing or not attached. Return the syringe and the package it came in to your pharmacy
- Do not remove the needle cap until just before you are ready to inject
- Do not touch the needle
- Do not try to put the cap back on the syringe
- Do not reuse the syringe
- Do not freeze or heat up the syringe
- Once removed from the refrigerator, do not store the syringe above 77ºF
- Do not expose to direct sunlight
- Do not inject through your clothes
- Do not use the pre-filled syringe if it has been damaged or if the needle cap is missing or not attached. Return the syringe and the package it came in to your pharmacy
- Do not remove the needle cap until just before you are ready to inject
- Do not touch the needle
- Do not try to put the cap back on the syringe
- Do not reuse the syringe
- Do not freeze or heat up the syringe
- Once removed from the refrigerator, do not store the syringe above 77ºF
- Do not expose to direct sunlight
- Do not inject through your clothes
This subcutaneous injection should be taken once every 2 weeks.
What you’ll need for your injection:
An unused KEVZARA pre-filled syringe
Alcohol wipe
Cotton ball or gauze
Sharps disposal container
Step A: Get ready for an injection
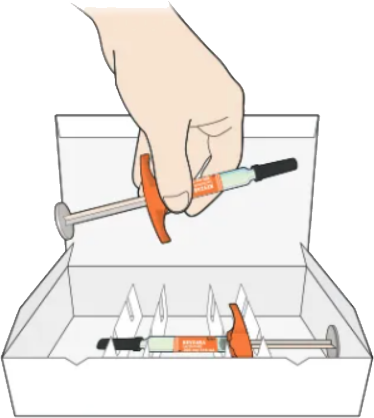
Take 1 syringe out of the package by holding the middle of the syringe body
Do not hold the syringe by the plunger, finger grip, or needle cap
Do not pull off the needle cap until you are ready to inject
Keep the remaining syringe in the carton in the refrigerator
Check that you have the correct medicine and the correct dose
Check the expiration date
Do not use the syringe if the expiration date has passed
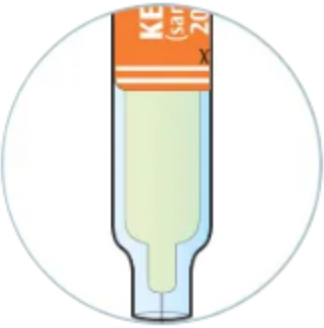
Check to see if the liquid in the syringe is clear and colorless to pale yellow
You may see air bubbles. This is normal
Do not use if the liquid is cloudy, discolored, or contains particles. Safely dispose of the syringe in a sharps container and get a new one

Using the syringe at room temperature may make the injection more comfortable
Do not use the syringe if it has been out of the refrigerator for more than 14 days
Do not try to warm the syringe in any other way
You can inject into the front of your thigh or your belly (abdomen), except for the area 2 inches (5 cm) around your belly button (navel)
If somebody else gives you the injection, they can also use the upper arm
Change the injection site each time you inject
Do not inject into skin that is tender, damaged, or has bruises or scars
Wash your hands
Clean the skin at the injection site with an alcohol wipe and let it air dry before injecting
Do not touch the injection site again before injection
Step B: Give the injection
(Complete Step B after completing all steps in Step A)
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.

Hold the syringe in the middle of the syringe body with the needle pointing away from you
Keep your hand away from the plunger
Do not get rid of any air bubbles in the syringe
Do not pull off the needle cap until you are ready to inject
Do not put the needle cap back on
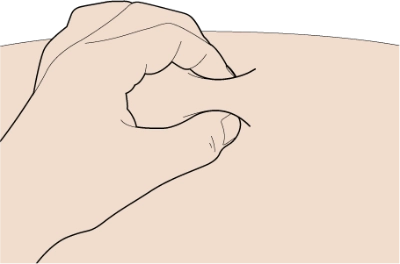
Use your thumb and first (index) finger to pinch a fold of skin at the cleaned injection site
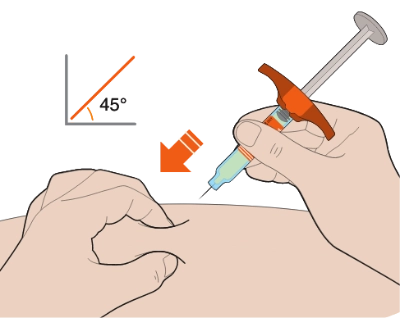
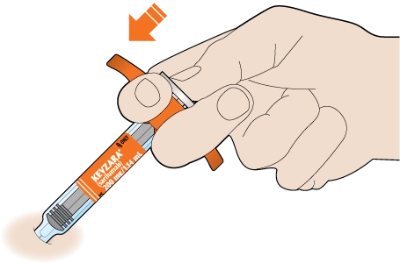
Slowly push the plunger down as far as it will go until the syringe is empty
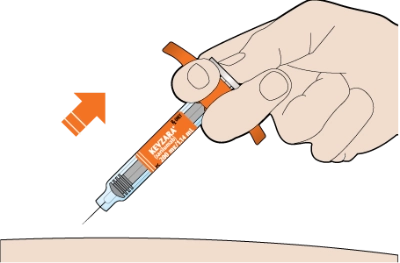
Pull the needle out at the same angle as inserted
If you see any blood at the injection site, press a cotton ball or gauze on the site
Do not rub the injection site
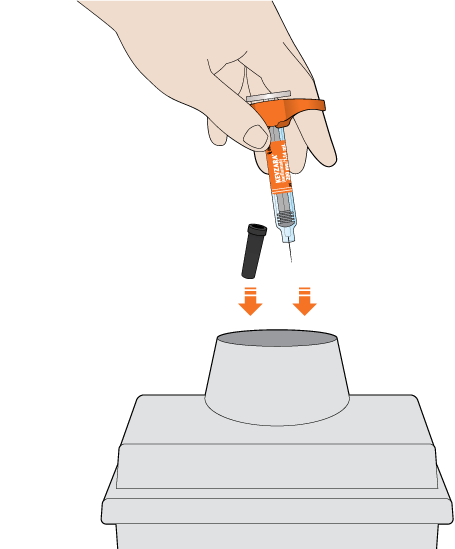
The KEVZARA syringe should not be reused
Put the used syringe into your FDA-cleared sharps disposal container or a puncture-resistant container
Do not put the needle cap back on
How should I dispose of (throw away) KEVZARA pre-filled syringes?
Put the used syringe in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the syringe in your household trash
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
Do not reuse the syringe.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Important: Always keep the sharps disposal container out of the reach of children.
Keep KEVZARA cool
When you fill your prescription for KEVZARA, it’s important to keep it in your refrigerator. But once it reaches room temperature, you’ll have to use the syringe within 14 days or throw it away.
IMPORTANT SAFETY INFORMATION
KEVZARA® (sarilumab) can cause SERIOUS SIDE EFFECTS including:
- SERIOUS INFECTIONS: KEVZARA is a medicine that affects your immune system. KEVZARA can lower the ability of your immune system to fight infections. Some people have had serious infections while using KEVZARA, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections. Your healthcare provider should test you for TB before starting KEVZARA. Your healthcare provider should monitor you closely for signs and symptoms of TB during treatment with KEVZARA.
- Before starting KEVZARA, tell your healthcare provider if you
- think you have an infection or have signs or symptoms of an infection, with or without a fever such as sweats or chills, muscle aches, a cough, shortness of breath, blood in your phlegm, weight loss, warm, red, or painful skin or sores on your body, diarrhea or stomach pain, burning when you urinate or urinating more often than normal, if you feel very tired, or if you are being treated for an infection, get a lot of infections or have repeated infections.
- have diabetes, HIV, or a weakened immune system.
- have TB, or have been in close contact with someone with TB.
- live or have lived, or have traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance of getting certain fungal infections (histoplasmosis, coccidioidomycosis, or blastomycosis).
- have or have had hepatitis.
- After starting KEVZARA, call your healthcare provider right away if you have any symptoms of an infection.
- CHANGES IN CERTAIN LABORATORY TEST RESULTS: Your healthcare provider should do blood tests before and after starting KEVZARA to check for low neutrophil (white blood cells that help the body fight off bacterial infections) counts, low platelet (blood cells that help with blood clotting and stop bleeding) counts, and an increase in certain liver function tests. Changes in test results are common with KEVZARA and can be severe. You may also have changes in other laboratory tests, such as your blood cholesterol levels. Your healthcare provider should do blood tests 4 to 8 weeks after starting KEVZARA and then every 6 months during treatment to check for an increase in blood cholesterol levels.
- TEARS (PERFORATION) OF THE STOMACH OR INTESTINES: Tell your healthcare provider if you have had a condition known as diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines. Some people using KEVZARA get tears in their stomach or intestine. This happens most often in people who also take nonsteroidal anti-inflammatory drugs (NSAIDS), corticosteroids, or methotrexate. Call your healthcare provider right away if you have fever and stomach (abdominal) pain that does not go away.
- CANCER: KEVZARA may increase your risk of certain cancers by changing the way your immune system works. Tell your healthcare provider if you have ever had any type of cancer.
- SERIOUS ALLERGIC REACTIONS: Serious allergic reactions can happen with KEVZARA. Get medical attention right away if you have any of the following signs: shortness of breath or trouble breathing; feeling dizzy or faint; swelling of your lips, tongue, or face; moderate or severe stomach (abdominal) pain or vomiting; or chest pain.
- Do not use KEVZARA if you are allergic to sarilumab or any of the ingredients of KEVZARA.
- Before using KEVZARA, tell your healthcare provider if you
- have an infection.
- have liver problems.
- have had stomach (abdominal) pain or a condition known as diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines.
- recently received or are scheduled to receive a vaccine. People who take KEVZARA should not receive live vaccines.
- All vaccines should be brought up-to-date before starting KEVZARA, unless urgent treatment initiation is required.
- plan to have surgery or a medical procedure.
- are pregnant or plan to become pregnant. It is not known if KEVZARA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Talk to your healthcare provider about the best way to feed your baby if you use KEVZARA. It is not known if KEVZARA passes into your breast milk.
- take prescription or nonprescription medicines, vitamins, or herbal supplements. It is especially important to tell your healthcare provider if you use:
- any other medicines to treat your PMR. Using KEVZARA with these medicines may increase your risk of infection.
- medicines that affect the way certain liver enzymes work. Ask your healthcare provider if you are not sure if your medicine is one of these.
- The most common side effects include:
- injection site redness
- injection site itching
- upper respiratory tract infection
- urinary tract infection
- nasal congestion, sore throat, and runny nose
These are not all the possible side effects of KEVZARA. Tell your doctor about any side effect that bothers you or does not go away. You are encouraged to report side effects of prescription drugs to the FDA at www.fda.gov/medwatch or call 1-800-FDA-1088.
To learn more, talk about KEVZARA with your healthcare provider or pharmacist. The FDA-approved Medication Guide and Prescribing Information can be found at www.KEVZARA.com or by calling 1-844-KEVZARA.
Please click here to see full Prescribing Information, including risk of SERIOUS SIDE EFFECTS, and Medication Guide.
What is KEVZARA?
KEVZARA is an injectable prescription medicine called an interleukin-6 (IL-6) receptor blocker. KEVZARA is used to treat adult patients with polymyalgia rheumatica (PMR) after corticosteroids have been used and did not work well or when a slow decrease in the dose of corticosteroids (taper) cannot be tolerated.
The health information contained herein is provided for general educational purposes only. Your healthcare provider is the single best source of information regarding your health. Please consult your healthcare provider if you have any questions about your health or treatment.
IMPORTANT SAFETY INFORMATION
KEVZARA® (sarilumab) can cause SERIOUS SIDE EFFECTS including:
- SERIOUS INFECTIONS: KEVZARA is a medicine that affects your immune system. KEVZARA can lower the ability of your immune system to fight infections. Some people have had serious infections while using KEVZARA, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections. Your healthcare provider should test you for TB before starting KEVZARA. Your healthcare provider should monitor you closely for signs and symptoms of TB during treatment with KEVZARA.
- Before starting KEVZARA, tell your healthcare provider if you
- think you have an infection or have signs or symptoms of an infection, with or without a fever such as sweats or chills, muscle aches, a cough, shortness of breath, blood in your phlegm, weight loss, warm, red, or painful skin or sores on your body, diarrhea or stomach pain, burning when you urinate or urinating more often than normal, if you feel very tired, or if you are being treated for an infection, get a lot of infections or have repeated infections.
- have diabetes, HIV, or a weakened immune system.
- have TB, or have been in close contact with someone with TB.
- live or have lived, or have traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance of getting certain fungal infections (histoplasmosis, coccidioidomycosis, or blastomycosis).
- have or have had hepatitis.
- After starting KEVZARA, call your healthcare provider right away if you have any symptoms of an infection.
- CHANGES IN CERTAIN LABORATORY TEST RESULTS: Your healthcare provider should do blood tests before and after starting KEVZARA to check for low neutrophil (white blood cells that help the body fight off bacterial infections) counts, low platelet (blood cells that help with blood clotting and stop bleeding) counts, and an increase in certain liver function tests. Changes in test results are common with KEVZARA and can be severe. You may also have changes in other laboratory tests, such as your blood cholesterol levels. Your healthcare provider should do blood tests 4 to 8 weeks after starting KEVZARA and then every 6 months during treatment to check for an increase in blood cholesterol levels.
- TEARS (PERFORATION) OF THE STOMACH OR INTESTINES: Tell your healthcare provider if you have had a condition known as diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines. Some people using KEVZARA get tears in their stomach or intestine. This happens most often in people who also take nonsteroidal anti-inflammatory drugs (NSAIDS), corticosteroids, or methotrexate. Call your healthcare provider right away if you have fever and stomach (abdominal) pain that does not go away.
- CANCER: KEVZARA may increase your risk of certain cancers by changing the way your immune system works. Tell your healthcare provider if you have ever had any type of cancer.
- SERIOUS ALLERGIC REACTIONS: Serious allergic reactions can happen with KEVZARA. Get medical attention right away if you have any of the following signs: shortness of breath or trouble breathing; feeling dizzy or faint; swelling of your lips, tongue, or face; moderate or severe stomach (abdominal) pain or vomiting; or chest pain.
- Do not use KEVZARA if you are allergic to sarilumab or any of the ingredients of KEVZARA.
- Before using KEVZARA, tell your healthcare provider if you
- have an infection.
- have liver problems.
- have had stomach (abdominal) pain or a condition known as diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines.
- recently received or are scheduled to receive a vaccine. People who take KEVZARA should not receive live vaccines.
- All vaccines should be brought up-to-date before starting KEVZARA, unless urgent treatment initiation is required.
- plan to have surgery or a medical procedure.
- are pregnant or plan to become pregnant. It is not known if KEVZARA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Talk to your healthcare provider about the best way to feed your baby if you use KEVZARA. It is not known if KEVZARA passes into your breast milk.
- take prescription or nonprescription medicines, vitamins, or herbal supplements. It is especially important to tell your healthcare provider if you use:
- any other medicines to treat your PMR. Using KEVZARA with these medicines may increase your risk of infection.
- medicines that affect the way certain liver enzymes work. Ask your healthcare provider if you are not sure if your medicine is one of these.
- The most common side effects include:
- injection site redness
- injection site itching
- upper respiratory tract infection
- urinary tract infection
- nasal congestion, sore throat, and runny nose
These are not all the possible side effects of KEVZARA. Tell your doctor about any side effect that bothers you or does not go away. You are encouraged to report side effects of prescription drugs to the FDA at www.fda.gov/medwatch or call 1-800-FDA-1088.
To learn more, talk about KEVZARA with your healthcare provider or pharmacist. The FDA-approved Medication Guide and Prescribing Information can be found at www.KEVZARA.com or by calling 1-844-KEVZARA.
Please click here to see full Prescribing Information, including risk of SERIOUS SIDE EFFECTS, and Medication Guide.
What is KEVZARA?
KEVZARA is an injectable prescription medicine called an interleukin-6 (IL-6) receptor blocker. KEVZARA is used to treat adult patients with polymyalgia rheumatica (PMR) after corticosteroids have been used and did not work well or when a slow decrease in the dose of corticosteroids (taper) cannot be tolerated.
The health information contained herein is provided for general educational purposes only. Your healthcare provider is the single best source of information regarding your health. Please consult your healthcare provider if you have any questions about your health or treatment.
MAT-US-2304906-v2.0-11/2025
Last Updated: November 2025